Chinese FDA approve Restore drug-coated balloon for in-stent restenosis and small vessel disease – BIBA Medtech Insights
Bard gets US FDA premarket approval for Lutonix 035 DCB in dysfunctional AV fistulae treatment - Interventional News

Benefit and risk from paclitaxel-coated balloon angioplasty for the treatment of femoropopliteal artery disease: A systematic review and meta-analysis of randomised controlled trials - eClinicalMedicine

FDA deems Orchestra BioMed's sirolimus-eluting balloon a breakthrough device for coronary restenosis | Fierce Biotech

Advanced NanoTherapies' SirPlux Duo Drug-Coated Balloon Receives FDA Breakthrough Designation for Small Vessel Coronary Artery Disease

Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group - ScienceDirect
MedAlliance's SELUTION SLR drug-eluting balloon (DEB) receives FDA investigational device exemption (IDE) approval, making it the first limus DEB to be available to US patients

Chocolate Touch drug-coated angioplasty balloon for treatment of PAD receives FDA approval - Vascular News
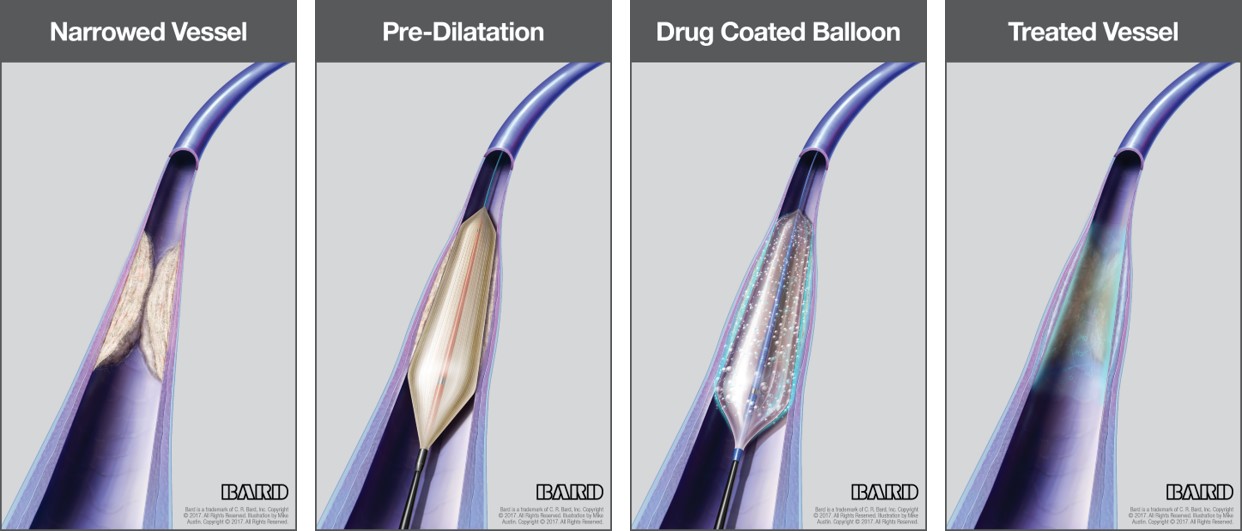
C. R. Bard Receives FDA Premarket Approval For The LUTONIX 035 Drug Coated Balloon - Medical Design and Outsourcing
Concept Medical received IDE approval to investigate safety and efficacy of its MagicTouch Sirolimus Coated Balloon Catheter for the treatment of small coronary artery disease | tctmd.com









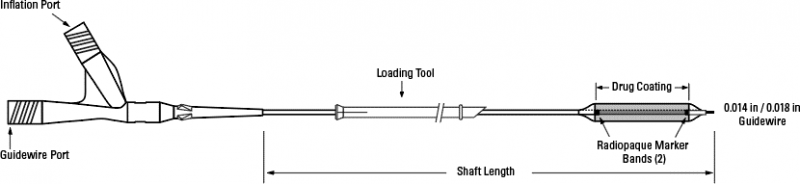

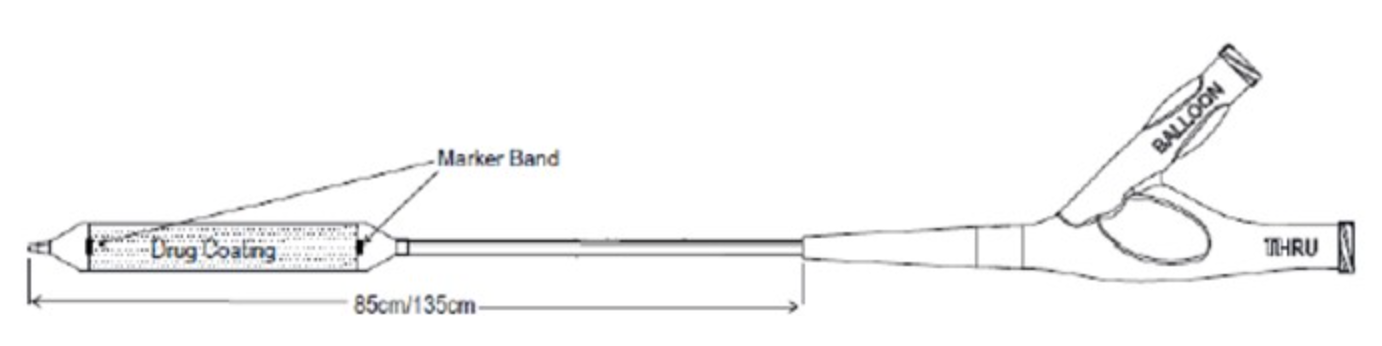
_1653668541.jpg)